Clinical Research & Medicines, Research & Preclinical Development
Hitting the “On Switch” in Hearing Loss
May 06, 2024
A novel gene therapy approach to switch on genetic drivers of hearing.
By: Meghan Drummond, PhD, Director of Auditory Sciences, Regeneron Genetic Medicines
Vassili Valayannopoulos, MD, PhD, MBA, Clinical Program Lead; Auditory Sciences, Regeneron Genetic Medicines
Updated February 20, 2025*
Vassili Valayannopoulos and Meghan (Meg) Drummond talk about Regeneron’s auditory program and the development of an investigational targeted gene therapy platform to potentially treat disorders like congenital hearing loss.

Vassili Valayannopoulos
The Impact of Sound
Sound is a significant part of the human experience, from within the womb, to birth and beyond. Think back to your earliest childhood memories: What do they sound like?
Hearing sounds is one of the key factors in early childhood development, allowing children to interact with and understand the world around them. Children born with severe hearing loss are unable to experience the sounds and spoken interactions that can help shape their development, potentially leading to difficulties in speaking, reading and forming social connections. Interventions, such as hearing amplification devices and learning sign language early on, can help to preserve and optimize language development.
Hearing loss impacts over 5% of the world’s population, including 34 million children.1 Some of these children are born with the condition, also known as congenital hearing loss. In the United States alone, up to 3 out of every 1,000 children are born with some degree of hearing loss.2 While hearing aids and cochlear implants can amplify sound to improve hearing for individuals with a range of hearing loss, these devices do not currently restore the full spectrum of sound. Imagine seeing the world in only 8 or 16 colors; although it may be better than black and white, it’s still not in “full color.”
I see a future where we can go so much further in addressing the needs of people with hearing loss, particularly early in their lives when it can have the biggest impact. A key to addressing these needs is understanding the underlying causes of hearing loss.
Approximately half of congenital hearing loss cases in the United States have genetic causes.2 Advances in genetic research have helped scientists and clinicians identify more than 6,000 variants in over 150 genes associated with hearing loss.3,4 That’s where we at Regeneron see the potential to change lives.
Fusion of Expertise
I recently joined Regeneron as the Clinical Program Lead for Auditory Sciences following the 2023 acquisition of Decibel Therapeutics, a biotechnology company founded by some of the world’s leading experts in hearing research.
Since 2017, experts in auditory biology and genetic medicines at Decibel and Regeneron have actively collaborated to build a rich pipeline of programs targeting congenital hearing loss caused by variants of known hearing loss genes. These include OTOF, as well as the more common GJB2 variant.2,4 Through this close partnership, we’ve realized the potential of applying our collective expertise to the ear, which is a great vehicle for discovery and therapeutic innovation for multiple reasons:
- Many genes involved in auditory disorders like hearing loss have been identified through studies with human genomic data and animal models, so we have definitive genetic targets for current and future therapeutic development.2,4
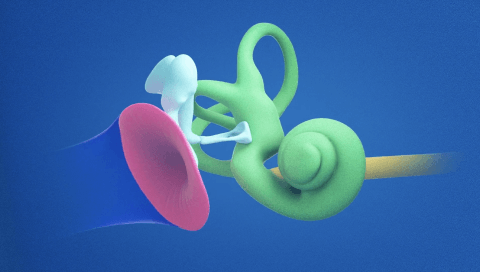
- We have methods of delivering medicines to the inner ear by administering directly into the cochlea (the part of the inner ear that sends signals to your brain in response to sound).3,5,6
- It is believed that the immune system has limited activity in the inner ear.6,7 The immune system functions to defend the body from foreign substances, even medicines, so delivering treatments to a relatively immune-privileged space may limit unintended systemic immune reactions.6,7

Understanding the Inner Ear and Our Approach to Genetic Medicines
As one team, we are deploying our exceptional scientific talent and proficiency in hearing loss, genetics expertise and extensive resources to collectively accelerate development of our genetic medicines portfolio for the auditory space.

Meghan (Meg) Drummond
Community Impact
I find the process by which we hear — the cellular biology, genetics and human impact — extremely fascinating and fulfilling to study. The field of hearing research and our understanding of how the inner ear functions at a molecular level has lagged behind many other organ systems because of the relative inaccessibility of the inner ear. However, over the last 20 to 30 years, huge gains in our understanding around the phenomenon of hearing have been driven by human genetics.
What’s so impactful about the work that we are doing now is that we’ve unlocked the potential to not only understand why a person has hearing loss, but to also develop potential therapeutics that could be life changing. It feels like we’re finally giving back to the community of deaf and hard-of-hearing individuals who have so graciously participated in studies with scientists, all so that we can gain a better understanding of hearing and make a difference. It’s truly a community achievement and what drives my work personally.
A Platform With Potential
Something unique about our approach to gene therapy in the inner ear is our use of cell-specific promoters. At a molecular level, every cell in the body has the same DNA (genes). However, sequences within that DNA that need to be “turned on” to produce the missing protein in the inner ear for hearing are different from those that get turned on in other parts of the body, or even other parts of the ear. Nature has a way to control this process using genetic elements called promoters. Promoters function as the “on switch” for genes that control certain biological processes such as protein expression in precisely the right cells in the body.
Our teams of skilled molecular biologists and geneticists at Regeneron have harnessed this specificity in creating our auditory gene therapy platform. By using inner ear cell-specific promoters, we are looking to activate our gene therapy in the cells of the body where it is required.
What Is Regeneron’s Auditory Gene Therapy Platform?
Multiple technologies that can serve as the foundation or starting point for different treatment applications.
Outside “vehicle”
Delivery capsule called a viral vector. The viral vector known as adeno-associated virus (AAV) can enter many different types of cells in the body to deliver its contents. They are non-disease-causing viruses that cannot self-replicate and can successfully enter the target cells for our auditory gene therapy.
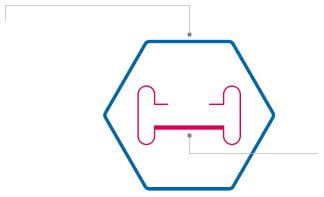
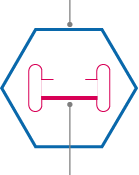
Inside “cargo”
Genetic material, such as DNA encoding our gene of interest. In the case of our auditory gene therapies, the genetic cargo includes a gene that is critical for normal hearing paired with a cell-specific promoter or “on switch” to activate this gene in certain cells in the ear.
Want to learn more? Check out the science behind genetic medicines.
Addressing Hearing Loss the Regeneron Way
I often get asked: How did your team arrive at this auditory gene therapy platform?
The answer: We approached hearing loss in much the same way we approach any therapeutic area at Regeneron. We start with the basic science — how the condition works genetically and biologically. Once we gain a deep understanding of the underlying problem, we can then carefully and confidently select the right tools for the right application.
We have seen our approach come to life from concept to clinic with encouraging early results: A gene therapy from Regeneron’s pipeline recently showed preliminary improved auditory responses in children with profound genetic hearing loss.
Beyond the Ear
Our work so far in the auditory space has hit on key features that can help shape the next generation of gene therapies: An AAV gene delivery system that we know will enter cells combined with promoters that turn on specific genes in specific cells. We hope our gene therapy platform will help flip the switch in the field of genetic medicines in general, potentially extending to other areas such as balance disorders, neurodegeneration, metabolic disorders and beyond.
All therapies discussed herein are investigational and have not been evaluated by any regulatory authority.
References
- World Health Organization. (2024). Deafness and hearing loss. https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss. (Accessed April 8, 2024)
- Renauld JM, Basch ML. Congenital deafness and recent advances towards restoring hearing loss. Curr Protoc. 2021;1(3):e76.
- Carpena NT, Lee MY. Genetic hearing loss and gene therapy. Genomics Inform. 2018;16(4):e20.
- Azaiez H, et al. Genomic Landscape and Mutational Signatures of Deafness-Associated Genes. Am J Hum Genet. 2018;103(4):484-497.
- Hahn R, et al. Gene Therapy for Inherited Hearing Loss: Updates and Remaining Challenges. Audio/ Res. 2023;13(6):952-966.
- Ishibashi V. et al. Immune responses in the mammalian inner ear and their implications for AAV-mediated inner ear gene therapy. Hear Res. 2023;432:108735.
- Hu BH, et al. Immune cells and non-immune cells with immune function in mammalian cochleae. Hear Res. 2018;362:14-24.
*This story has been updated from when it was first published to reflect our latest perspectives.